Brief Overview Gum turpentine is a fluid obtained by the distillation of resin obtained from trees, mainly pine trees. It is composed of terpenes, mainly monoterpenes alpha-pinene and beta-pinene. It has a paint-like odor and an appearance of transparent clear oily liquid that is water white to slightly yellow in color. The word turpentine is formed (via French and Latin) from the Greek word terebinthine, the name of a species of tree, the terebinth tree, from whose sap the spirit was originally distilled. It is used as a source of raw materials in the synthesis of fragrant chemical commercial compounds such as camphor, linalool, alpha-terpineol, and geraniol. These products are usually produced from alpha-pinene and beta-pinene. The components can be further separated via steam distillation, fractional distillation, and extractive distillation. The origins of turpentine can be traced back to ancient civilizations, including those in Egypt, Greece, and China. It is believed that the Egyptians were among the first to utilize pine resin in embalming processes, taking advantage of its preserving properties. In ancient Greece, it was used as incense in religious ceremonies, owing to its pleasant aroma and perceived spiritual significance. Throughout history, turpentine has been used for medicinal purposes. In ancient times, it was employed as a topical treatment for various ailments, including wounds and skin infections. The resin's antiseptic and antibacterial properties made it a valuable remedy for ancient healers. During the Roman Empire, the compound became an important commodity. It was primarily sourced from the vast pine forests in southern Spain. Roman traders exported this material to different regions of the empire, expanding its use and popularity across Europe. In the Middle Ages, this commodity continued to be a sought-after material. It was used in religious ceremonies, as well as for medicinal and aromatic purposes. During the Renaissance period, this commodity gained significance in the burgeoning art scene. Artists used it as a solvent for their oil-based paints, enabling them to create masterpieces with vibrant colors and smooth textures. The colonial era in America saw the rise of the naval stores industry, which centered around pine resin extraction. Longleaf pine forests in the southeastern United States became valuable sources of turpentine and rosin (a solid residue derived from the distillation of turpentine). The naval stores industry played a vital role in early American trade and commerce, providing essential materials for shipbuilding and preservation. The 19th century witnessed significant advancements in industrial processes, leading to a surge in demand for the material. It became a crucial ingredient in the production of paints, varnishes, and adhesives, revolutionizing various industries. The advent of steam-powered distillation techniques allowed for more efficient extraction of turpentine and rosin, further fueling its commercial use. During the 19th and early 20th centuries, the compound's aromatic qualities were celebrated in the art world. Artists and writers expressed their fascination with its scent, associating it with nature and nostalgia. The product's presence in art studios and its use by renowned painters contributed to its romanticized image in cultural circles. Turpentine is a solvent for many alkyd resins and has more solvency than mineral spirits or odorless mineral spirits. Its high solvent strength makes it the best choice for thinning oils and natural resins. It is considered to be a better solvent than mineral spirits, and the best solvent for natural resins, such as dammar and mastic also it readily dissolves most of the natural varnish resins. It is recommended for artists' painting or varnish applications over other products. Chemical intermediates are compounds produced from raw materials that serve as building blocks for the synthesis of more complex chemicals or products. In this context, turpentine is used as a starting material to derive a range of valuable chemical compounds. In the chemical industry, it is used as a source of raw materials in the synthesis of fragrant chemical commercial compounds such as camphor, linalool, alpha-terpineol, and geraniol. These products are usually produced from alpha-pinene and beta-pinene. Traditional oil-based paints and varnishes contain turpentine as a fundamental ingredient. One of the most important benefits of it in paint and varnish is its ability to speed up the drying process. As the solvent evaporates, it allows the paint to dry relatively quickly, reducing the time between application and the final product's use or display. This rapid drying time is particularly advantageous for artists who may need to layer or rework their artwork without waiting for extended periods between coats. By using turpentine as a medium, artists can achieve smooth gradations and transitions between colors. Additionally, it serves as a thinner, which helps to create transparent glazes or adjust the paint's consistency for various artistic techniques. In varnishes, this compound contributes to the glossy finish that many artists and woodworkers desire. As the varnish dries, the turpentine evaporates, leaving behind a clear and shiny protective coat that enhances the beauty of the underlying surface. In addition to its use as a thinner, it can increase the transparency of paint. When mixed with pigments, it allows the colors to maintain their vibrancy while also creating a more translucent effect when applied to the canvas. As a natural extract from pine trees, turpentine offers a unique and authentic fragrance. This natural aspect appeals to consumers seeking products with fewer synthetic ingredients and a connection to nature. In perfumery, fragrances are typically classified into three main categories of notes - top notes, middle notes, and base notes. Turpentine falls into the middle note category. It adds depth and complexity to a fragrance and serves as a bridge between the initial top notes and the long-lasting base notes. In addition to the applications mentioned above, turpentine has other uses in many sectors such as medicine, aromatherapy, household cleaning products, etc which could be explored in detail on our Blog pageGENERAL DESCRIPTION OF GUM TURPENTINE
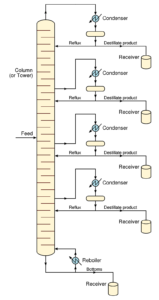
Gum Turpentine Manufacturing Process




Origins and Early Uses
Roman Trade
Middle Ages and Renaissance
Colonial America and Naval Stores
19th Century Industrialization
Presence in Art and Culture
Solvent Industry
Chemical Intermediates
Paint & Varnish Industry
Fragrance & Perfume Industry
PRODUCT SPECIFICATION PRODUCT IDENTIFICATION Synonyms : terpene, dipanol, gum spirits, turps. CAS No.: : 8006-64-2 Molar mass : 136 g/mol Chemical Formula : C10H6 PHYSICAL AND CHEMICAL PROPERTIES MELTING POINT : -60 ~ -50 °C Boiling Point : 50-180 °C SALES SPECIFICATION APPEARANCE : transparent, anhydrous, no foreign matter, no suspension Color : Not deeper than water-white & water-clear Density D20/4 : 0.870 max Refractive Index N20/4 : 1.4670-1.4710 Initial Distilling Point °C : 150 min Distilling Volume below 170°C : 90 % Acid Value mgKOH/g : 0.5 max TRANSPORTATION PACKING : in galvanized iron drum of about 150/175kgs net each, 80drums/14mt or 120drums/18mt per 20’ft container. Must be kept away from heat and flame. HAZARD CLASS : 3.3 UN NO. : 1299